Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2024, vol. 23(3), pp. 205–209 DOI: https://doi.org/10.15388/LietChirur.2024.23(3).7
Metaplastic Carcinoma Breast in Polycythemic Octogenarian – Lessons Learnt: Case Report
Swarup Prabhu
5 Air Force Hospital, Jorhat, Assam, India
E-mail: swarupprb@gmail.com
Munish Malhotra
Department of Surgery, Command Hospital Air Force Bangalore, Bengaluru, India
E-mail: drmunishmalhotra@gmail.com
https://ror.org/05cx69s52
Naresh Saidha
Department of Surgery, Command Hospital Air Force Bangalore, Bengaluru, India
E-mail: nareshkumarsaidha@gmail.com
https://ror.org/05cx69s52
Ritu Mehta
Command Hospital Air Force Bangalore, Bengaluru, India
E-mail: doctorritumehta@gmail.com
https://ror.org/05cx69s52
Surjeet Dwivedi
Command Hospital Air Force Bangalore, Bengaluru, India
E-mail: surjeetdwivedi@gmail.com
https://orcid.org/0000-0001-6595-0718
https://ror.org/05cx69s52
Tarun Mohan Gupta
Department of Surgery, Command Hospital Air Force Bangalore, Bengaluru, India
E-mail: tmgupta26@gmail.com
https://ror.org/05cx69s52
Abstract. An 80-year-old lady presented with lump Left breast of 8 months duration. Clinically was aT4b lesion, sonomammogram showed BIRADS V lesion and core needle breast biopsy features were suggestive of metaplastic carcinoma breast of adenosquamous type, ER, PR-Positive, Her2Neu-Negative. Patient received preoperative hormonal therapy followed by modified radical mastectomy and postoperative adjuvant chemotherapy and hormonal therapy. Post operative patient showed good clinical recovery and was recurrence free at 6 months follow up although patient had all the poor prognostic factors with large size, LN involvement and high Ki 67. This article is a rare example of metaplastic breast carcinoma presenting in geriatric age group and showing good recovery despite presence of all poor prognostic factors. Hence this report is to enlighten the medical caregivers about the existence of this rare entity and challenges involved in management of this entity.
Keywords: metaplastic carcinoma breast, geriatric population, polycythemia, poor prognosis.
Received: 2024-06-16. Accepted: 2024-07-08.
Copyright © 2024 Swarup Prabhu, Munish Malhotra, Naresh Saidha, Ritu Mehta, Surjeet Dwivedi, Tarun Mohan Gupta. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Metaplastic breast carcinoma (MpBC) is an exceedingly rare heterogeneous histologic subtype of breast cancer accounting for less than 1% of all breast cancers [1]. It is characterized by the histological presence of more than 2 cellular types, commonly a mixture of epithelial and mesenchymal components. This variant harbours a triple-negative breast cancer (TNBC) phenotype and has a worse prognosis and decreased survival compared to TNBC [2]. The term “metaplastic carcinoma” was first published by Huvos and colleagues in 1973 [3]. The WHO Classification of Breast Tumours classifies MpBC as mixed metaplastic carcinoma, low-grade adenosquamous carcinoma, fibromatosis-like, squamous cell carcinoma, spindle cell carcinoma, and metaplastic carcinoma with mesenchymal differentiation [4]. Most of these variants are aggressive and chemo resistant and have a high propensity to metastasize, except fibromatosis-like carcinoma and low-grade adenosquamous carcinoma [5]. This case report brings out a rare case of metaplastic carcinoma breast in an 80-year-old female. We document our difficulties and lessons learnt in managing this case.
Case report
An 80-year-old female k/c/o HTN, COPD, hypothyroidism, and JAK 2 mutation positive polycythaemia vera presented with lump left breast of 8 months duration. No history suggestive of locoregional or distant metastases. On examination 6x4 cm lump retro areolar region, irregular surface, hard in consistency, fixed to breast tissue and overlying skin, not fixed to the chest wall. No palpable axillary lymphadenopathy, examination of right breast was normal. Sono mammogram showed Lobulated hypoechoic lesion measuring 4.4x3.2x4.7 cm from 5 to 7 o’clock region in retro and periareolar region – BIRADS-V. Two clustered areas of pleomorphic calcifications in upper outer and central quadrants – BIRADS-4b (Figure 1). F18 FDG PET CT scan showed large heterogeneously enhancing FDG avid soft tissue mass with spiculated margins measuring 46x42x43 mm (max SUV – 13.2) in retro areolar region extending to lower quadrant. The lesion is seen abutting the overlying skin, the planes with underlying muscles maintained. No abnormal enhancing or FDG avid lesion in brain, lungs, liver, adrenal or skeleton (Figure 2). USG guided Core needle biopsy was done which revealed metaplastic carcinoma of adenosquamous type. ER, PR-positive, HER 2 Neu-Negative.
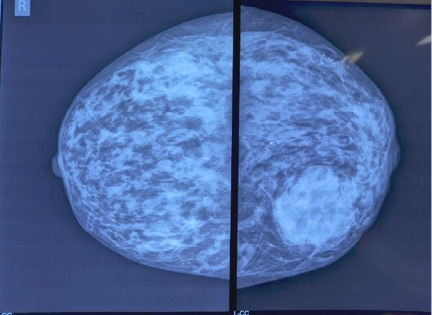
Figure 1. Mammographic findings both breasts
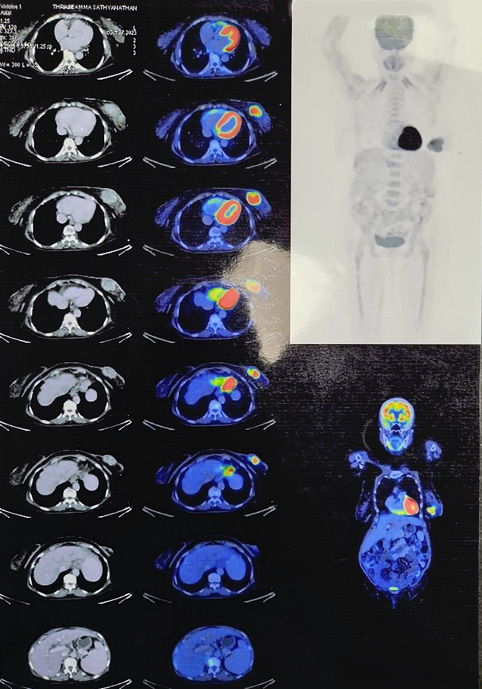
Figure 2. PET CT images showing large heterogeneously enhancing FDG avid soft tissue mass with spiculated margins measuring 46x42x43 mm (max SUV – 13.2)
Patient received hormonal therapy with letrozole (aromatase inhibitors) for 2 months prior to surgery. For polycythemia vera she underwent multiple episodes of therapeutic phlebotomies. Underwent modified radical mastectomy left under GA. Intraop-T4b lesion involving retroareolar region, few enlarged left axillary LNs present.
Histopathological examination revealed Metaplastic carcinoma breast – squamous cell carcinoma (>90% tumour cells), moderately differentiated Grade II (Figure 3). No lymphovascular or perineural invasion seen. 2/25 lymph nodes positive for metastasis. pT4bN1aMx ER-positive in DCIS (Figure 4d), PR & HER 2 neu-Negative, P63-positive in squamous compartment (Figure 4b), Ki67–90% (Figure 4c).
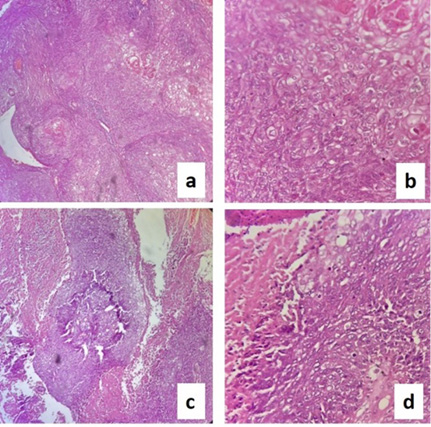
Figure 3. HPE slides showing: a) squamous cell carcinoma (SCC) 10x; b) SCC 40x; c) SCC with necrosis 10x; d) SCC with necrosis 40x
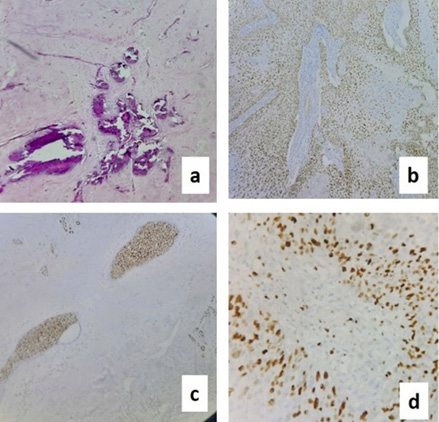
Figure 4. Slides showing: a) calcification; b) P63 positive in SCC; c) Ki67 positive 90%; d) ER positive in DCIS
Post operatively managed with adjuvant chemotherapy, 6 cycles of paclitaxel and carboplatin and hormonal therapy with letrozole was continued. The patient showed good clinical response and is doing well with no evidence of local or systemic recurrence at 6 months follow up.
Discussion
According to a large study by Pezzi et al. [6] mean age of MpBC was 61.1 years with higher prevalence in African-American and Hispanic patients. Our patient was an 80-year-old elderly female and only few cases have been reported in this age group. Metaplastic carcinomas pose a diagnostic dilemma due to their complex morphologic subtypes. The differential diagnoses can overlap with those of breast sarcomas and phyllodes tumours. Therefore, accurate immunohistochemistry stains are required as these disease entities differ in prognosis and treatment approach [7]. MpBC has a lower rate of axillary lymph node metastasis compared to the invasive breast carcinoma of the same size, and late distant metastasis can be seen without lymph node involvement [8]. Due to its rarity, the treatment guidelines are still uncertain for MpBC. Despite its worse prognosis and treatment challenges, there are no current, specific therapeutic guidelines for MpBC patients. They are usually treated more aggressively than patients with ductal or lobular carcinoma with mastectomy and adjuvant chemotherapy, in accordance with international guidelines depending on hormonal receptor, Her2 status and TNM stage. Many studies have shown that the choice of appropriate chemotherapy regime depends on the histological type of metaplastic breast. Cases with a squamous epithelial component responded to cisplatin-based chemotherapy regimens, while cases with sarcomatous elements showed good response to doxorubicin and ifosfamide-based regimens [9]. Using immunohistochemical stains in our patient p63, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and Ki-67 status were assessed. Our patient was fitting into triple negative group although ER was positive in DCIS component. HER2 was also negative and Ki-67 was very high (90%). Considering this our patient received preoperatively hormonal therapy with letrozole followed by modified radical mastectomy and post operatively adjuvant chemotherapy with paclitaxel and carboplatin 6 cycles and showed good clinical response. According to study by Hu et al. [10] in 1 665 patients of MpBC disease-related survival improved with adjuvant radiotherapy, especially in patients with triple negative disease. Considering the advanced age group radiotherapy was not given as decided by tumour board of the institution. Tumor size larger than 5.0 cm, lymph node involvement and Ki-67 ≥14% indicate a poor prognosis in patients with metaplastic breast carcinoma [11]. Although all the above parameters were positive our patient showed good clinical response with no locoregional or distant metastasis at 6 months follow up.
Conclusion
MpBC is a very rare, has unique features, both clinical and pathological, which differentiates it from ductal carcinoma of the breast and can be challenging to diagnose both clinically and histopathologically. Early diagnosis is important to achieve better patient outcomes. The specific characteristics of this entity requires personalized treatments as decided by a tumour board with multidisciplinary team members. Immunohistochemistry, plays an important role in diagnosis and classification of subtypes. Molecular pathways involved in the development MpBC will guide in better understanding and development of individualized therapeutic options. However, the data about MpBC is still limited and lack of a more specific therapeutic approach is an unmet need that warrants further research and randomized clinical trials to improve their overall patient outcome and survival.
References
1. Thapa B, Arobelidze S, Clark BA, Xuefei J, Daw H, Cheng YC, Patel M, Spiro TP, Haddad A. Metaplastic breast cancer: characteristics and survival outcomes. Cureus 2022; 14(8): e28551. DOI: 10.7759/cureus.28551.
2. Reddy TP, Rosato RR, Li X, Moulder S, Piwnica-Worms H, Chang JC. A comprehensive overview of metaplastic breast cancer: clinical features and molecular aberrations. Breast Cancer Research 2020; 22: 121. DOI: 10.1186/s13058-020-01353-z.
3. Huvos AG, Lucas JC Jr, Foote FW Jr. Metaplastic breast carcinoma. Rare form of mammary cancer. N Y State J Med 1973; 73(9): 1078–1082.
4. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ (Eds.). WHO classification of tumours of the breast, 4th edition, vol. 4. Geneva, Switzerland: WHO Press, 2012
5. McMullen ER, Zoumberos NA, Kleer CG. Metaplastic breast carcinoma: update on histopathology and molecular alterations. Arch Pathol Lab Med 2019; 143(12): 1492–1496.
6. Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol 2007; 14(1): 166–173.
7. Erjan A, Almasri H, Abdel-Razeq H, Al-Masri M, Haddad H, Alnsour A, Rahman FA, Dayyat A. Metaplastic breast carcinoma: experience of a tertiary cancer center in the Middle East. Cancer Control 2021; 28: 10732748211004889. DOI: 10.1177/10732748211004889.
8. Shah DR, Tseng WH, Martinez SR. Treatment options for metaplastic breast cancer. ISRN Oncology 2012; 2012: 706162.
9. Papatheodoridi A, Papamattheou E, Marinopoulos S, Ntanasis-Stathopoulos I, Dimitrakakis C, Giannos A, Kaparelou M, Liontos M, Dimopoulos MA, Zagouri F. Metaplastic carcinoma of the breast: case series of a single institute and review of the literature. Med Sci 2023; 11(2): 35. DOI: 10.3390/medsci11020035.
10. Hu J, Zhang H, Dong F, Zhang X, Wang S, Ming J, Huang T. Metaplastic breast cancer: treatment and prognosis by molecular subtype. Translational Oncology 2021; 14(5): 101054. DOI: 10.1016/j.tranon.2021.101054.
11. Song Y, Liu X, Zhang G, Song H, Ren Y, He X, Wang Y, Zhang J, Zhang Y, Sun S, Liang X, Sun Q, Pang D. Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. World J Surg Oncol 2013; 11: 129. DOI: 10.1186/1477-7819-11-129.